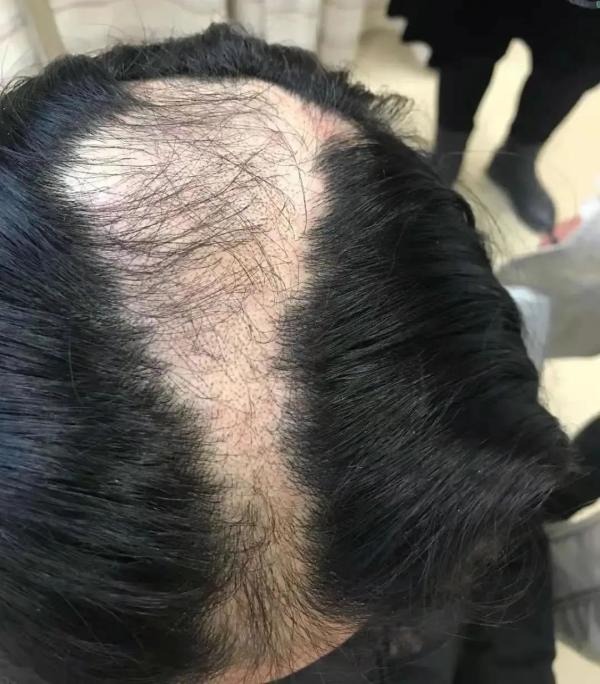
斑秃福音:突破性疗法认定花落礼来JAK
斑秃又一项突破性疗法认定花落礼来JAK
2020年3月16日,礼来制药与Incyte联合宣布,美国FDA授予Baricitinib(巴瑞替尼)突破性疗法资格认定(Breakthrough Therapy designation),用于治疗斑秃(alopecia areata ,AA)。突破性疗法认定(BTD)旨在加快可能用于治疗严重疾病的药物开发和审评,前提是初步临床证据表明该药物可能在具有临床意义的终点上表现出相对于现有疗法的实质性改善。
获此认定基于针对斑秃的2/3期BRAVE-AA1临床研究的积极结果。该研究在成人斑秃患者中评估了巴瑞替尼与安慰剂的疗效与安全性。在BRAVE-AA1的2期研究中,直至36周,未报告新的安全性信号和严重不良事件。
斑秃(alopecia areata ,AA)影响着500-700万美国人;雄性秃发影响着大约5000万美国男性,约3000万美国女性。据不完全统计,2018年中国共有377万的斑秃患者。
斑秃主要特征为头部、脸部和身体其他部位整块脱发。通常认为斑秃是一种T细胞介导的累及毛囊的器官特异性自身免疫性疾病,其发病机制涉及遗传学、自身免疫学、精神因素和微量元素缺乏等,但其确切病因及分子机制尚不明确。
若斑秃( alopecia areata,AA)发生在头部,从前俗称鬼剃头。
斑秃通常发生的部位以及年龄分布:
斑秃潜在的治疗方案:(从局部到全身性,从轻度到重度)
药融圈数据统计,全球范围内有多款JAK 抑制剂正在开展斑秃适应症:巴瑞替尼 (Baricitinib)、辉瑞 PF-06651600 (BTD)目前处于 3 期临床试验;其他的有芦可替尼(Ruxolitinib)、托法替布(Tofacitinib)、PF-06700841、CTP-543(氘代Ruxolitinib)、ATI-502等等。
国内开展的有泽璟制药的的杰克替尼、恒瑞医药/瑞石生物的SHR0302。
礼来巴瑞替尼在国内启动3期国际多中心临床
附礼来原文:
INDIANAPOLIS,March 16, 2020/PRNewswire/ --Eli Lilly and Company(NYSE: LLY) andIncyte Corporation(NASDAQ: INCY) announced today that theU.S. Food and Drug Administration(FDA) has granted Breakthrough Therapy designation to baricitinib for the treatment of alopecia areata (AA), an autoimmune disorder that can cause unpredictable hair loss on the scalp, face and other areas of the body. The Breakthrough Therapy designation aims to expedite the development and review of drugs that are intended to treat a serious condition when preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over already available therapies on a clinically significant endpoint(s).
"Patients with AA currently do not have any FDA-approved treatment options available to them," said Lotus Mallbris, M.D., Ph.D., vice president of immunology development atLilly. "AA not only causes hair loss but also may be a psychosocial burden for people living with this disease. AtLilly, we aspire to create new medicines that can give hope to patients. We look forward to working with the FDA to further explore baricitinibs potential to become the first approved treatment option for these individuals."
The FDA Breakthrough Therapy designation is based on the positive Phase 2 results ofLillys adaptive Phase 2/3 study BRAVE-AA1, which evaluated treatment with baricitinib versus placebo in adult patients with AA. In the Phase 2 portion of the BRAVE-AA1 study up to Week 36, there were no new safety signals with no serious adverse events reported. The reported treatment-emergent adverse events (TEAEs) were mild or moderate and the most common included upper respiratory tract infections, nasopharyngitis and acne.
Based on the interim results of the Phase 2 part of the study, the Phase 3 portion of BRAVE-AA1 and an additional Phase 3 double-blind study (BRAVE-AA2), are currently assessing the efficacy and safety of the 2-mg and 4-mg doses of baricitinib relative to placebo.
"There are millions of people around the world affected by and living with AA," saidDory Kranz, president and CEO of theNational Alopecia Areata Foundation. "Were encouraged by baricitinibs potential to be one of the first FDA-approved medicines to treat AA."
Baricitinib is currently approved for the treatment of adults with moderately to severely active rheumatoid arthritis (RA). More than 100,000 patients have experience using baricitinib, which is approved in over 65 countries including theU.S., member states of the EU andJapan. It is marketed as OLUMIANT®.
友情提示:专业医疗咨询,药品购买请前往专业医院或者药店。
参考:
NMPA/CDE;
药融圈数据;
FDA/EMA/PMDA;
相关公司公开披露;
https://www.lilly.com/;
https://www.lilly.com/news/press-releases;
https://www.naaf.org/alopecia-areata;
https://investor.lilly.com/news-releases/news-release-details/lilly-receives-fda-breakthrough-therapy-designation-baricitinib;
等等。
RECOMMEND
本周推荐阅读
药融圈致力于打造医药行业生态圈,共建、共创、共赢、共享,旨在解决生物医药发展过程中的共性关键问题(项目、资本、人才等),将平台的协同、联动、共享、共赢优势最大化实现并发挥,惠及千万企业。对大企业实现加速赋能,对中小企业实现助力孵化,形成企业服务领域新生态。药融圈围绕我国生物医药产业链,针对生物医药大数据、技术和资本投资、药融园(产业园)等开展系列系统性工作,促进我国生物医药产业健康发展,完善产业链,共同面对全球合作和竞争。